Abstract
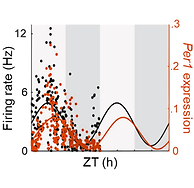
The brain’s biological clock, the suprachiasmatic nucleus (SCN), exhibits endogenous 24-hour rhythms in gene expression and spontaneous firing rate; however, the functional relationship between these neuronal rhythms is not fully understood. Here, we used a Per1::GFP transgenic mouse line that allows for the simultaneous quantification of molecular clock state and firing rate in SCN neurons to examine the relationship between these key components of the circadian clock. We find that there is a stable, phased relationship between E-box-driven clock gene expression and spontaneous firing rate in SCN neurons and that these relationships are independent of light input onto the system or of GABAA receptor-mediated synaptic activity. Importantly, the concordant phasing of gene and neural rhythms is disrupted in the absence of the homologous clock gene Per1, but persists in the absence of the core clock gene Per2. These results suggest that Per1 plays a unique, non-redundant role in phasing gene expression and firing rate rhythms in SCN neurons to increase the robustness of cellular timekeeping.