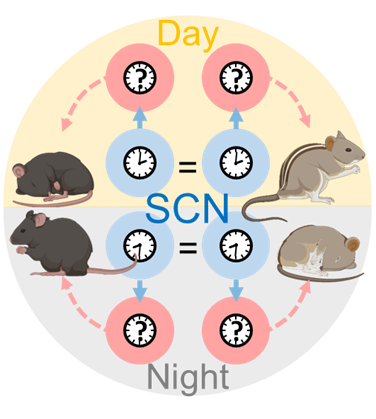
In this project, we test our lab’s central hypothesis using two complementary species, the night-active laboratory mouse and the day-active African striped mouse. Critically, SCN rhythms peak at the same time of day in each species, suggesting that temporal niche preference is organized downstream from the SCN. We propose that SCN signals must be differentially encoded by neurons in the dorsomedial hypothalamus that subsequently generate appropriately-timed, species-specific behavioral rhythms.
In this project, we aim to revolutionize the way circadian biologists study behavior. “Behavioral rhythms” are almost exclusively wheel-running rhythms, but these are only a subset of an animal’s behavioral repertoire. To overcome this, we use machine learning to automatically identify circadian rhythms in multiple behaviors simultaneously. We have used this method to measure sex- and estrogen-dependent differences in circadian behavior and are now investigating how rhythms change in the context of social interactions.
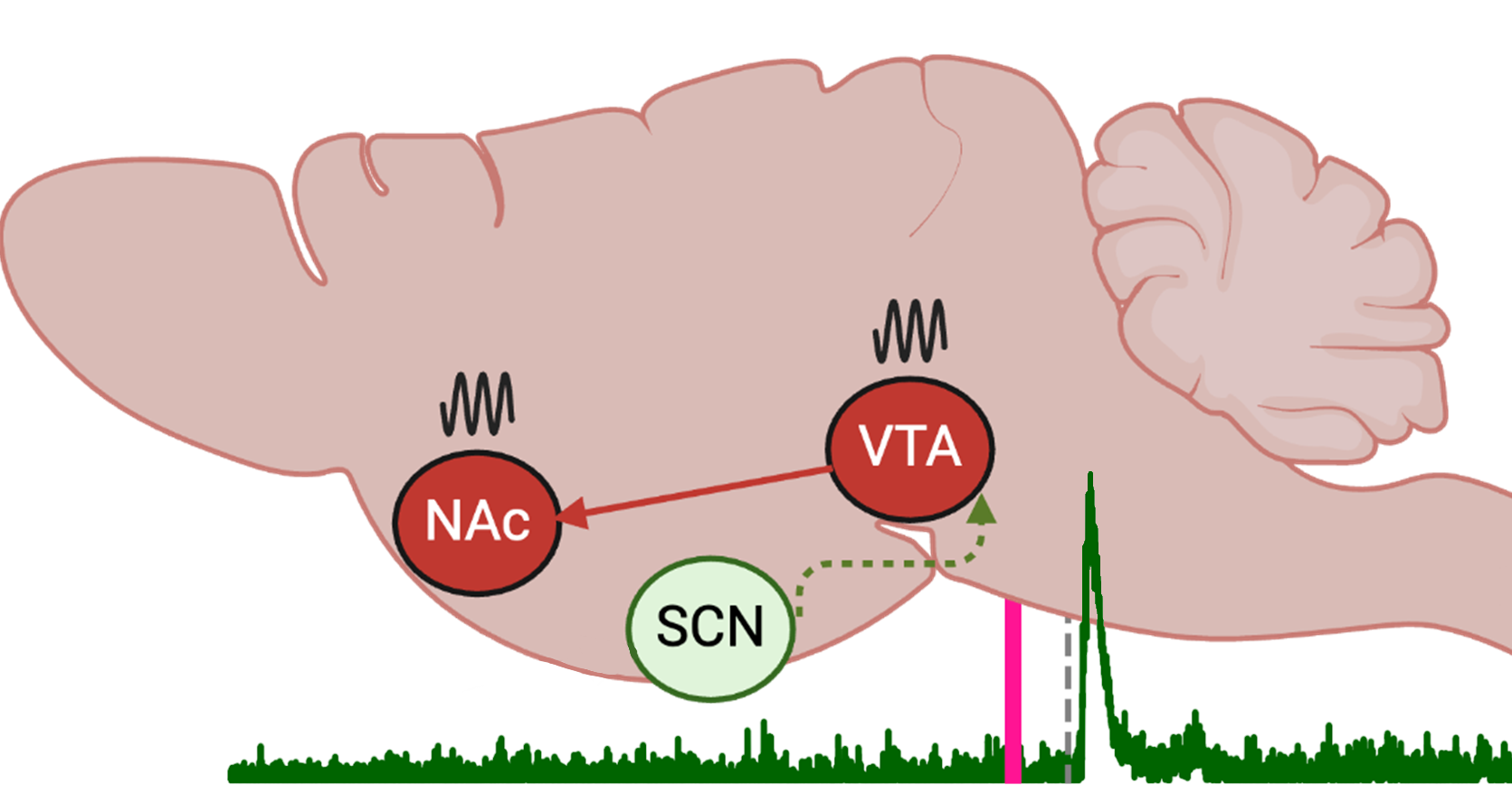
In this project, we explore how chronic stress influences circadian rhythms in the release of dopamine, a neurotransmitter crucial for motivated behavior. Loss of motivation is a central feature of major depressive disorder, and depression has been linked to circadian clock dysfunction in the mesolimbic dopamine pathway. We propose that disruption of the circadian clock due to chronic stress attenuates the rhythmic release of dopamine in the nucleus accumbens, driving the emergence of depressive phenotypes.
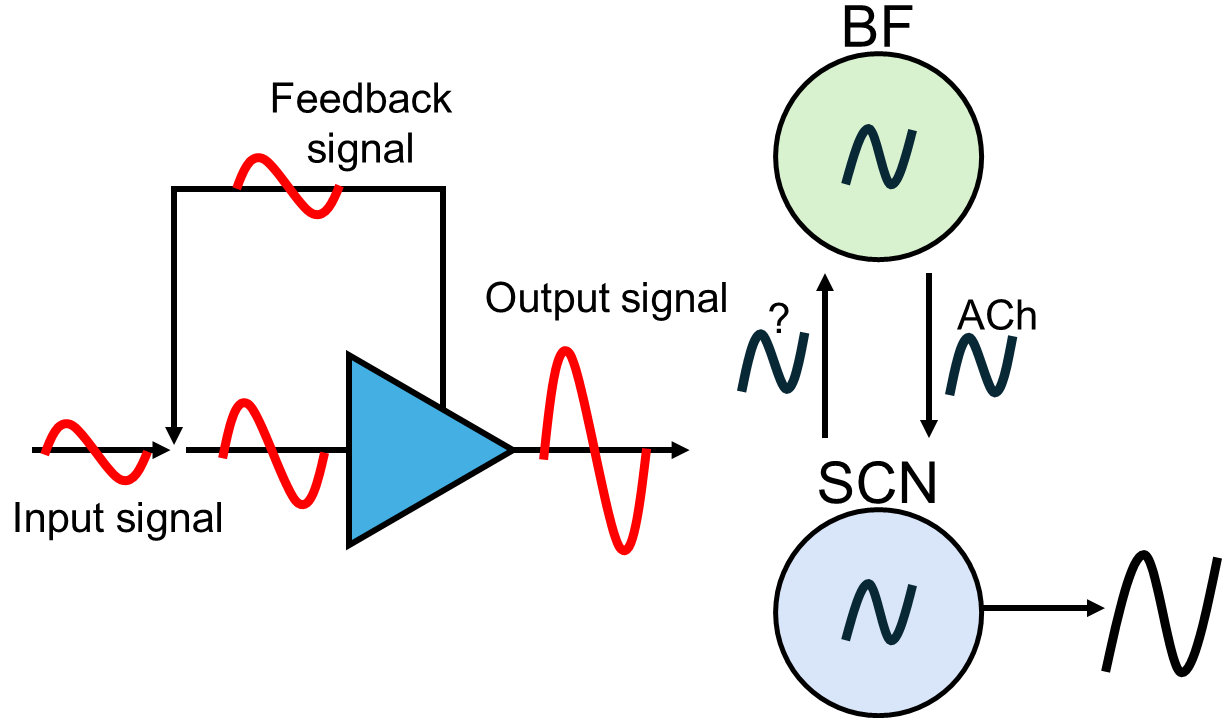
In this project, we (in collaboration with Dr. Jun Wang, TAMHSC) test the hypothesis that a cholinergic feedback loop from the SCN, to the basal forebrain, and back is necessary to reinforce circadian rhythms in behavior and physiology. We propose that rhythmic SCN input to the basal forebrain weakens with age and in diseases such as Alzheimer’s, inhibiting cholinergic feedback to the SCN, which weakens the SCN signal even more. This leads to a breakdown of the feedback loop and, consequently, health impairment.
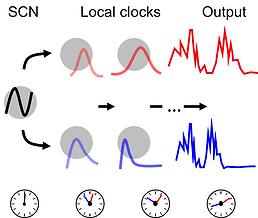
We are broadly interested in understanding circadian output circuitry. Other questions we are focusing on include (but are not limited to): defining the “transfer function” that takes place in SCN target neurons and determining the role of local clocks in this encoding process, identifying how rhythms in the brain reflect hormone differences in circadian outputs and how brain clocks are regulated by hormone release, and determining how the circadian system integrates SCN input and noise and if particular input neurons are more robust to noise than others.